EAST PROVIDENCE – IlluminOss Medical Inc.’s Photodynamic Bone Stabilization System, which relies on light-sensitive glue to achieve a speedy and less-intrusive fracture repair, has undergone its first human trial, according to a report in Medical News Today.
“This case represents a major milestone for IlluminOss and patients worldwide,” said Robert A. Rabiner, the company’s founder and CEO, who was honored in 2007 as the Rhode Island Innovator of the Year. (READ MORE) “We believe that our minimally invasive technology will fundamentally improve the treatment of hundreds of thousands of bone fractures by driving much earlier restoration of functionality, improving the durability of orthopedic repairs, decreasing pain and reducing scarring.”
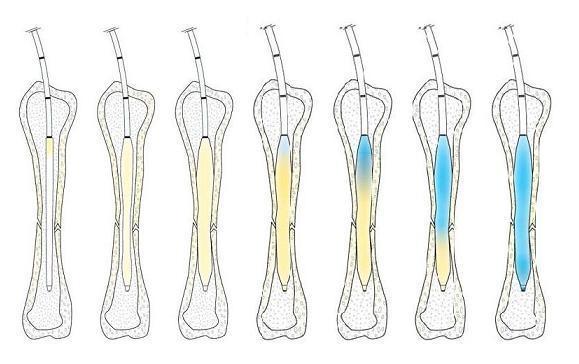
The IlluminOss Photodynamic Bone Stabilization System – intended to eliminate the need for stabilization of fractures with the traditional pins, plates and screws – requires an incision only 4.5 millimeters in diameter. The process involves inserting a balloon catheter, along with a light-transmitting optical fiber. The catheter is filled with a light-curable compound, to fit the contours of the bone, then the light is turned on to rapidly harden the polymer.
Many details of the case were withheld to protect patient confidentiality. But the company did report that the patient was a 21-year-old male with a fracture to the fifth metacarpal: the long bone in the hand that attaches to the pinky finger.
“The instrumentation is intuitive and easy to use,” Dr. Arnold-Peter C. Weiss, the company’s chief medical officer, told Medical News Today. “The case report from the hospital indicates that the overall procedure went according to plan, with no complications.
“The patient had mobility immediately. At six weeks, the x-rays showed an advanced consolidation process, with no migration of the device and no displacement of the fracture. Clinically, the patient had complete mobility and almost the same strength with a minimal scar. He was working and leading a normal life.”
IlluminOss Medical Inc. is a privately-held early-stage medical company developing minimally-invasive systems for the stabilization and treatment of bone fractures. The company last year announced it had raised $11.2 million to finance clinical trials of the system. (READ MORE) Additional information is available at www.IlluminOss.com.












