EAST PROVIDENCE – IlluminOss Medical Inc. today announced it has been granted a CE mark for its minimally invasive Photodynamic Bone Stabilization System, allowing the fracture-repair system to be sold in the European Union.
Traditional repair methods often require that a large incision be made, so plates and screws can be inserted to stabilize the fracture, the company noted. “There may be extensive soft tissue damage; the patient has limited or no mobility initially, which may lead to temporary or permanent stiffness; and the patient may experience severe pain,” the firm said.
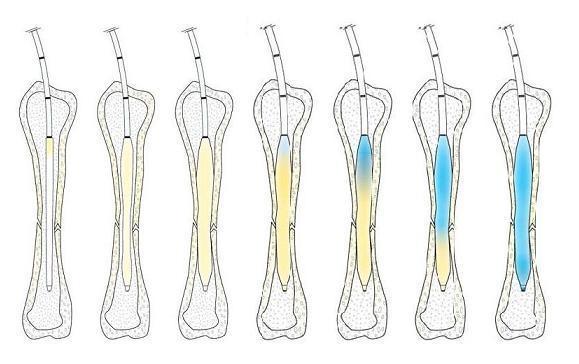
By contrast, the IlluminOss Photodynamic Bone Stabilization System “requires just a small, 4.5-milimeter pathway into the bone.” A slender balloon catheter is inserted, along with a light-conducting fiber; the balloon is then filled with a light-sensitive liquid, taking on the contours of the bone; and once the proper configuration is achieved, the light source is activated, hardening the liquid in about 90 seconds.
The system – which recently had its first human trial (READ MORE) – was designed “to enable the patient to have immediate joint mobility,” the company said. By eliminating or minimizing the need to immobilize the adjacent joints, it “minimizes the potential for tendon adhesions and a tenolysis procedure to remove them,” IlluminOss said. Moreover, soft-tissue injury caused by the repair is “virtually eliminated,” because the incision is so small.
The granting of a CE mark indicates that a product meets EU consumer safety, health or environmental requirements. The mark is required on many classes of products – including machinery and toys – before they can be sold in the EU or the broader European Economic Area (EEA).
“This approval represents a major milestone for IlluminOss and patients in the EU,” said Robert A. Rabiner, the company’s founder and president, who was honored by PBN in 2007 as the Rhode Island Innovator of the Year. (READ MORE)
“We believe that our minimally invasive technology will fundamentally improve the treatment of hundreds of thousands of bone fractures by driving much earlier restoration of functionality, improving the durability of orthopedic repairs, decreasing pain and reducing scarring.”
He said the company has begun the process of selecting an EU distribution partner.
IlluminOss Medical Inc. is a privately-held early-stage medical company developing minimally-invasive systems for the stabilization and treatment of bone fractures. The company last year announced it had raised $11.2 million to finance clinical trials of the system. (READ MORE) Additional information is available at www.IlluminOss.com.











